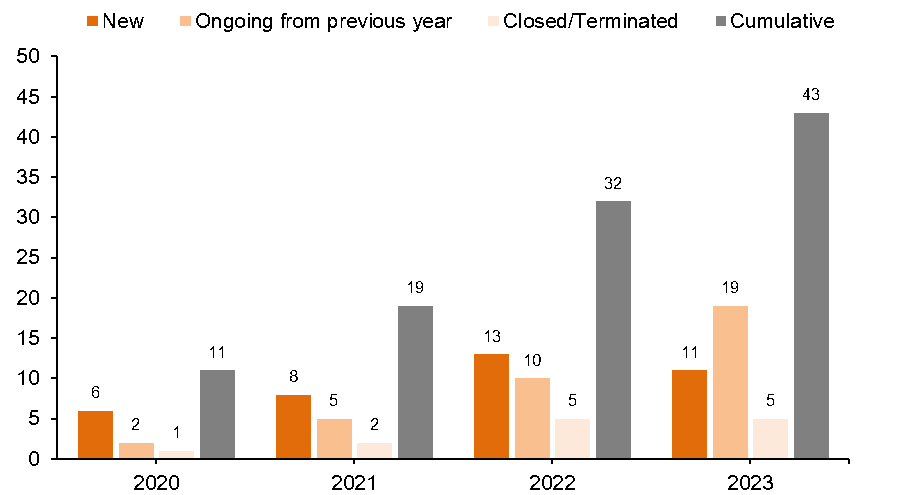About Clinical Research Centre
Sunway Medical Centre’s Clinical Research Centre (SunMed CRC) commenced in August 2009 in response to the demand for evidence-based practice as well as SunMed’s wish to participate significantly in medical research. CRC is dedicated primarily towards coordinating all research involving human subjects. Such research studies may be related to:
- pharmaceutical products,
- medical devices,
- medical radiation and imaging,
- surgical procedures,
- medical records,
- biological samples,
- and/or epidemiological, social and psychological investigations.
CRC seeks to promote ethical and reliable clinical research according to the international and local Good Clinical Practice (GCP) standards. We bridge communication between investigators and their counterparts; and assist with feasibility assessments conducted by interested parties. Apart from facilitating SunMed to secure research projects, we assist in documenting and tracking progress of the clinical researches conducted. In response to the demand for evidence-based clinical research, we seek to increase participation of SunMed and to contribute significantly to the health and research industry.
VISION
To be the leading and pioneering centre of excellence in ASEAN region for research in a private hospital.
MISSION
To create a sustainable research ecosystem in Sunway Medical Centre by enabling medical staff and professionals to learn and discover with the goal to provide the best patient care.
GOAL
To set up a Clinical Research Centre (CRC) for the conduct of quality clinical trials and research according to Good Clinical Practice (GCP) for the advancement of medicine and to improve Quality of Life for the patients.
OBJECTIVES
- Set up a strong governance structure within Clinical Research Centre (CRC) to facilitate communication, approval process and quality research conducts which benefits and improves the quality of life for the patients.
- To set up comprehensive infrastructure and facilities to support research.
- Foster strong research culture among consultants, allied healthcare partners and nursing staffs, and establish research identity.
- Establish SunMed as partner of choice for research collaboration among CROs, Industry, Academia & Community.
SERVICES
Clinical Research Centre (CRC), established within Sunway Medical Centre, is essential to the conduct of clinical research initiated in SunMed and therefore the facilities and resources are made available to meet the needs of sponsors/contract research organization (CRO), investigators, and all subjects. Such services include:
- Feasibility Studies
CRC assists consultants to review feasibility of protocols received from pharmaceutical companies or CROs, taking into consideration of the resources, patient pool, patient indication, facilities and services required in conducting the study in an institution. - Ethics Review
We have an institutional committee, the Sunway Medical Centre Independent Research Ethics Committee (SREC) constituted of medical professionals and non-medical members, whose responsibility is to ensure the rights, safety and well-being of human subjects involved in a clinical trial. It is compulsory for all studies involving human subjects at SunMed to be reviewed and approved by SREC for conduct. - Legal Review
We have in-house legal advisor that review the provisions of the agreements involved. - Pre-Clinical Trial Organising and Planning
CRC reviews the trial design and procedures to ensure that SunMed has the expertise and facilities to conduct the relevant procedures. CRC liaises and discusses with relevant departments and consultants on the protocol procedures to ensure any concerns are dealt with efficiently. - Clinical Trial Operations
Managing key deliverable issues, personnel, data, finance and operational matters in line with hospital policies to meet sponsor/CRO’s, GCP and regulatory requirements. - Site initiation and closure
We provide venue for site initiation and closure. CRC ensures that all pre-initiation and initiation procedures are performed accordingly to meet GCP and regulatory requirements.
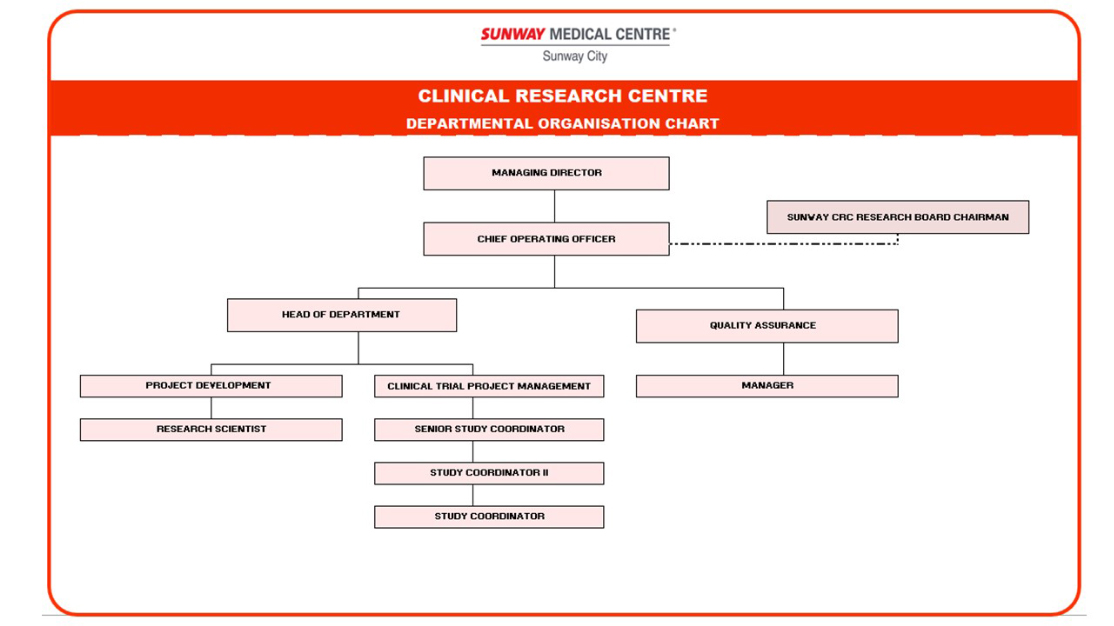
ORGANIZATION CHART
INDUSTRY SPONSORED RESEARCH (ISR)
Industry Sponsored Research from the year 2020 -2023
Active Clinical Trials
The list of clinical trials that are currently active in recruiting participants and pending site initiation at Sunway Medical Centre. For those who are interested in participating in the clinical trials, please contact us.
| 1 | KontRASt-02: A randomized, controlled, open label, phase III study evaluating the efficacy and safety of JDQ443 versus docetaxel in previously treated subjects with locally advanced or metastatic KRAS G12C mutant non-small cell lung cancer | Participant with locally advanced or metastatic (stage IIIB/IIIC or IV) KRAS G12C mutant non-small cell lung cancer | Recruiting Participant |
| 2 | A Phase II Study to Assess the Efficacy and Safety of 5 Years of Osimertinib in Patients with EGFR Mutation Positive Stage IB-IIIA Non-small Cell Lung Carcinoma, Following Complete Tumour Resection (TARGET) | Participant with non-small cell lung carcinoma (stage IB-IIIA) | Recruiting Participant |
| 3 | MANIFEST-2: A Phase 3, Active-Control Study of Pelabresib (CPI0610) and Ruxolitinib vs. Placebo and Ruxolitinib in JAKi Treatment Naive MF Patients (MANIFEST-2) | Participant diagnosed with Myelofibrosis (Treatment Naive) | Recruiting Participant |
| 4 | A Phase IIIb of Tolerability and Efficacy of Oral Asciminib Versus Nilotinib in Patients with Newly Diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase | Participant with Philadelphia Chromosome Positive Chronic Myeloid Leukaemia (in Chronic Phase) | Recruiting Participant |
| 5 | A Phase 2b to Assess the Efficacy and Safety of Orally Administered NS-018 versus Best Available Therapy in Subjects with Primary Myelofibrosis, Post-Polycythemia Vera Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis with Severe Thrombocytopenia (Platelet Count <50,000/μL) | Participant diagnosed with Myelofibrosis, with severe thrombocytopenia (platelet count <50,000/μL) | Recruiting Participant |
| 6 | A Study to Evaluate the Safety and Efficacy of the Unity-b Biodegradable Balloon-expandable Biliary Stent System in Subjects with Biliary Strictures | Participant with symptomatic biliary strictures suffering from jaundice and associated with dark urine, pale stools, and pruritus. Patients with previous PTBD and/or stent who need secondary stenting for prolonged symptom relief | Recruiting Participant |
| 7 | Efficacy and Safety of M281 in Adults with Warm Autoimmune Hemolytic Anemia: A Multicenter, Randomized, Double-blind, Placebo-controlled Study with a Long-term Open-label Extension | Participant diagnosed with Warm Autoimmune hemolytic anemia (wAIHA) | Recruiting Participant |
| 8 | SPADE - aSia Pacific Avelumab bladder cancer maintenance | Participant diagnosed with Bladder Cancer | Recruiting Participant |
| 9 | A Phase III Study to Evaluate the Efficacy and Safety of Tozorakimab in Patients Hospitalised for Viral Lung Infection Requiring Supplemental Oxygen (TILIA) | Participant with viral lung infection requiring supplemental oxygen | Recruiting Participant |
| 10 | A Phase 3b, Placebo-controlled Study Evaluating the Efficacy and Safety of Subcutaneously Administered Guselkumab in Improving the Signs and Symptoms and Inhibiting Radiographic Progression in Participants with Active Psoriatic Arthritis. (APEX) | Participant with active Psoriatic Arthritis | Recruiting Participant |
| 11 | A Phase IIb/Phase III study to evaluate the efficacy and safety of spesolimab in patients with moderate to severe hidradenitis suppurativa. Lunsayil 1 | Participant with skin condition known as Hidradenitis suppurativa (HS) | Recruiting Participant |
| 12 | EvasayilTM: A placebo-controlled trial to evaluate the efficacy and safety of spesolimab in the treatment of patients with Netherton syndrome | Participant with Netherton syndrome (NS) with confirmed diagnosis of NS (SPINK5 causative mutations) at baseline, aged 12 years and older with weight minimum 35 kg | Recruiting Participant |
| 13 | A Phase I/III Study to Evaluate the Safety and Neutralizing Activity of AZD5156/AZD3152 for Pre exposure Prophylaxis of COVID 19 in Participants with Conditions Causing Immune Impairment | Participant who are Immunocompromised and seeking for COVID 19 protection | Recruiting Participant |
| 14 | A Post-marketing Trial (in select countries) to Evaluate Efficacy and Safety and The Impact of Immunogenicity on Efficacy, Safety, and Pharmacokinetics of Spesolimab I.V. in Treatment of Patients with Generalized Pustular Psoriasis Presenting with a Recurrent Flare Following Their Initial GPP Flare Treatment with Spesolimab I.V. | Participant with Generalized Pustular Psoriasis (GPP) | Recruiting Participant |
| 15 | A Phase 3, Placebo-controlled Trial to Evaluate the Efficacy and Safety of Sibeprenlimab Administered Subcutaneously in Subjects with Immunoglobulin A Nephropathy (Otsuka) | Participant with chronic kidney disease (CKD) | Recruiting Participant |
| 16 | A Phase 3, Placebo-Controlled Study to Evaluate the Safety, Immunogenicity, and Efficacy of V181 Dengue Vaccine in Healthy Participants 2 to 17 Years of Age | Participants who are 2 to 18 years old and are seeking immunization against dengue disease | Recruiting Participant |
| 17 | A 2-Part, Open-Label, Phase 2, Multiple Dose Study to Evaluate the Pharmacodynamic Effects, Safety, and Tolerability of Patiromer in Children under 6 Years of Age with Hyperkalaemia (EMERALD 2) | Male and female infants, toddlers, and children from birth to <6 years of age with hyperkalaemia | Pending Site Initiation (March 2024) |
| 18 | A Phase 3b Study to Assess the Efficacy, Safety, and Tolerability of Remibrutinib 25 mg B.I.D. in Comparison to Placebo with Omalizumab 300 mg Every 4 Weeks as Active Control Over 52 Weeks in Adult Patients with Chronic Spontaneous Urticaria Inadequately Controlled by Second-generation H1-antihistamines. | Participant with Chronic Spontaneous Urticaria | Pending Site Initiation |
| 19 | Phase 3b study to assess the efficacy, safety, and tolerability of Remibrutinib 25 mg b.i.d. in comparison to placebo with Omalizumab 300 mg every 4 weeks as active control over 52 weeks in adult patients with chronic spontaneous urticaria inadequately controlled by second-generation H1-antihistamines | Participant with Chronic Spontaneous Urticaria (CSU) or hives | Pending Site Initiation |
| 20 | A Phase III of Savolitinib in Combination with Osimertinib Versus Platinum-Based Doublet Chemotherapy in Participants with EGFR Mutated MET-Positive, Locally Advanced or Metastatic Non-Small Cell Lung Cancer Who Have Progressed Following Treatment with Osimertinib (SASAFFRON) | Participants with EGFR Mutated MET-Positive, Locally Advanced or Metastatic NSCLC | Pending Site Initiation |
INVESTIGATOR INITIATED RESEARCH (IIR)
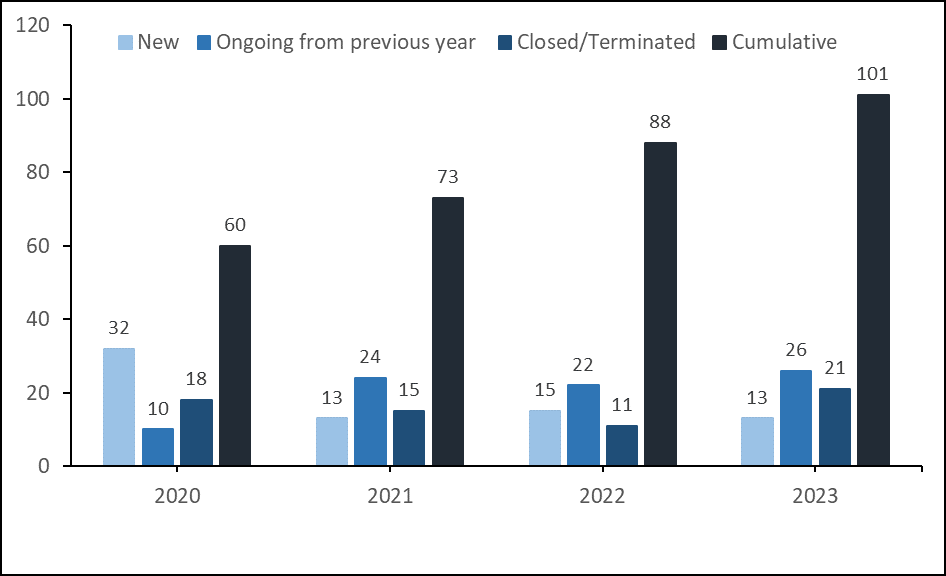
Investigator Initiated Research (IIR) from the year 2020-2023
RESEARCH OUTPUT
Top 5 most cited research output. For the full list of research outputs, please click the link here.

CRC Policies and Procedures
|
Policy number |
Policy name |
Applicability |
|---|---|---|
|
SMC-SOS-CORP-CRC-001 |
Scope of Services |
IIR |
|
SMC-GL-CORP-CRC-001 |
Prepare for an Audit or Inspection |
IIR |
|
SMC-GL-CORP-CRC-002 |
External Grant Application for Investigator Initiated Research (IIR) |
IIR |
|
SMC-GL-CORP-CRC-003 |
Grand Management for Investigator Initiated Research (IIR) |
IIR |
|
SMC-SOP-CORP-CRC-007 |
Request for Research collaboration with SunMed |
IIR |
|
SMC-SOP-CORP-CRC-008 |
SunMed Funding Application |
IIR, IISR |
|
SMC-SOP-CORP-CRC-009 |
Document Control for research Related Documents |
ISR, IISR |
|
SMC-SOP-CORP-CRC-010 |
Protocol Development |
IISR (if applicable), IIR |
|
SMC-SOP-CORP-CRC-013 |
Informed Consent Process |
IIR, IISR, ISR |
|
SMC-SOP-CORP-CRC-016 |
Re-consent process |
IIR, IISR, ISR |
|
SMC-SOP-CORP-CRC-017 |
Taking Informed Assent Process |
IIR, IISR, ISR |
|
SMC-SOP-CORP-CRC-019 |
Research Team Training |
IIR, IISR, ISR |
|
SMC-SOP-CORP-CRC-020 |
Handling of Research Noncompliance |
IIR, ISR, IISR |
|
SMC-SOP-CORP-CRC-021 |
Assisting in Site Initiation Visit for ISR |
ISR |
|
SMC-SOP-CORP-CRC-022 |
Assisting in (COV) for Industry Sponsored Research (ISR) |
ISR |
|
SMC-SOP-CORP-CRC-023 |
Assisting in Archiving in (ISR) |
ISR |
|
SMC-SOP-CORP-CRC-027 |
Safety Surveillance Management |
ISR, IISR |
|
SMC-SOP-CORP-CRC-028 |
Research Sample Management |
ISR |
|
SMC-SOP-CORP-CRC-029 |
Submitting Study Progress Report & End of Research Report |
IIR |
|
SMC-SOP-CORP-CRC-030 |
Laboratory Specimen Handling & Shipment in ISR |
ISR |
|
SMC-SOP-CORP-CRC-031 |
Investigational Product (IP) Management |
ISR, IISR |
|
SMC-SOP-CORP-CRC-032 |
Investigational Product (IP) Dispensing |
ISR, IISR |
|
SMC-SOP-CORP-CRC-033 |
Study Closeout for Investigator Initiated Research (IIR) |
IISR (If applicable) |
|
SMC-SOP-CORP-CRC-034 |
Site Initiation for Investigator Initiated Research (IIR) |
IIR |
|
SMC-SOP-CORP-CRC-035 |
Maintaining Study Documentation & Record Keeping in Industry Sponsored Research |
ISR, IISR |
|
SMC-SOP-CORP-CRC-036 |
Monitoring of Investigator Initiated Research (IIR) |
IIR |
|
SMC-SOP-CORP-CRC-037 |
Handling Feasibility Studies |
ISR |
|
SMC-SOP-CORP-CRC-038 |
Research Misconduct |
ISR, IISR, IIR |
|
SMC-SOP-CORP-CRC-039 |
Pre -Publication Submission Review |
IISR |
- Request for policies or Standard Operating Procedures, please write to: Head of Department, Clinical Research Centre at [email protected] .
- SunMed employee may access CRC policies and procedures via shared point.
- Applicant shall read through the relevant policies and procedures prior to the commencement of your research project.
About SunMed Independent Research Ethics Committee (SREC)
The Sunway Medical Centre Independent Research Ethics Committee (SREC) is a committee established under the authority of Sunway Medical Centre Berhad (SunMed). It evaluates the ethical aspects of research in SunMed, ensuring that they are reliably conducted in accordance with both international and Malaysian standards of ‘Good Clinical Practice’ (GCP).
SREC holds as its primary responsibility the safe-guarding of the rights, safety, and well-being of all research subjects. As per GCP requirements, members of the SREC are drawn from medical and non-medical sectors to ensure sufficient objectivity and independence from SunMed clinicians undertaking the research. The Clinical Research Centre (CRC) is the Secretariat to SREC
Registered with National Pharmaceutical Regulatory Agency (NPRA)
Sunway Medical Centre Independent Research Ethics Committee (SREC) is one of the 3 private hospital among the 13 Ethics Committee Registered with Drug Control Activity (DCA)
Objective
The objectives of SREC are to
- protect the rights, safety, dignity and wellbeing of the subjects of research
- promote ethical principles in human research
- provide special attention to research involving vulnerable subjects and use of genetic testing
- facilitate ethical research through efficient and effective review processes
- provide competent review and monitoring of human research projects whilst they are active
- promote awareness of the ethical conduct of human research
Scope
The scope of the SREC’s operations manual is applicable to all members of SREC and persons conducting research in SunMed. The scope also encompasses the following:
Terms of reference of the SREC which include:
- responsibilities,
- membership requirements,
- terms of appointment,
- liability coverage,
- conditions of appointment,
- offices, quorum & meeting requirements,
- use of independent panel and
- education of members.
- procedure for submitting an application to the SREC for
- approval to conduct research projects or to make amendments to a previously SREC approved research,
- Reviewing of applications by SREC,
- decision-making and the communication of decisions of the SREC,
- following up and monitoring of approved research,
- SREC documentation and archiving.
SREC Member (Term 2024 - 2025)
|
Name |
Gender |
Earned Degree |
Primary Specialty |
Occupation |
Affiliation With SunMed |
Status |
|---|---|---|---|---|---|---|
|
Assoc Prof Dr Quek Kia Fatt (Chairperson) |
M |
PhD, FRIPH |
Scientific |
Lecturer of Community Health, Jeffrey Cheah School of Medicine & Health Sciences |
No |
Re-elected |
| Dato’ Dr Chang Kian Meng (Vice-Chairperson) | M | MBBS, MRCP, FRCP, FRCPA (Haem) | Scientific |
Consultant Haematologist & Transplant Physician |
Yes |
Re-elected |
|
Mr Edwin Tan (Secretary) |
M |
BSc |
Scientific |
Senior Manager, CRC |
Yes |
Re-elected |
|
Dr Michael Wong Pak Kai |
M |
MBChB (Sheffield), M.Med Surgery (USM), AM (Malaysia), JCMT (Japan), Fellowship in Colorectal Surgery (Thailand & Malaysia) |
Scientific |
Colorectal Surgeon, General Surgery |
Yes |
New |
|
Assoc Prof Dr Hafizah Zainuddin |
F |
MBBS (IIUM), MPaeds (UM) |
Scientific |
Consultant Dermatologist |
No |
New |
| Dr Thangesweran Ayakannu | M |
MBBS (Trinidad, West Indies), MLiP (UK), DFFP (UK), MRCOG (UK), PhD (UK) |
Scientific |
Consultant Obstetrics & Gynaecology -Oncologist |
Yes |
Extended |
| Dr Jennifer Leong Siew Mooi | F |
MBBS (IMU), MCO (UM) |
Scientific |
Consultant Clinical Oncologist |
Yes |
Extended |
|
Ms Azliana Binti Abu Bakar Sajak |
F |
BSc, MSC |
Scientific |
Research Scientist |
Yes |
Extended |
|
Prof Dr Abhi Veerakumarasivam |
M |
PhD (Oncology & Genetics) |
Scientific |
Professor, Department of Medical Sciences |
No |
Extended |
|
Ms Chia Ee Vy |
F |
BSc in Pharmacy |
Scientific |
Research Pharmacist |
Yes |
Extended |
| Mr Ashwin A/L Kumar |
M |
LL.B |
Non-Scientific |
Senior Manager, Legal Services |
Yes |
Extended |
|
Ms Amy Khoo |
F |
LL.B |
Non-Scientific |
Director, Legal Services |
Yes |
Extended |
|
Mr Wong Mun Fai |
M |
BEng |
Non-Scientific (Layman) |
Civil Engineer |
No |
Extended |
|
Ms Priyadarshini Subramaniam |
F |
LL.B |
Non-Scientific (Layman) |
Lawyer |
No |
Extended |
|
Nor Atiqah Ahmad Nordin |
F |
BSc |
Non-Voting Member |
Quality Assurance |
Yes |
New |
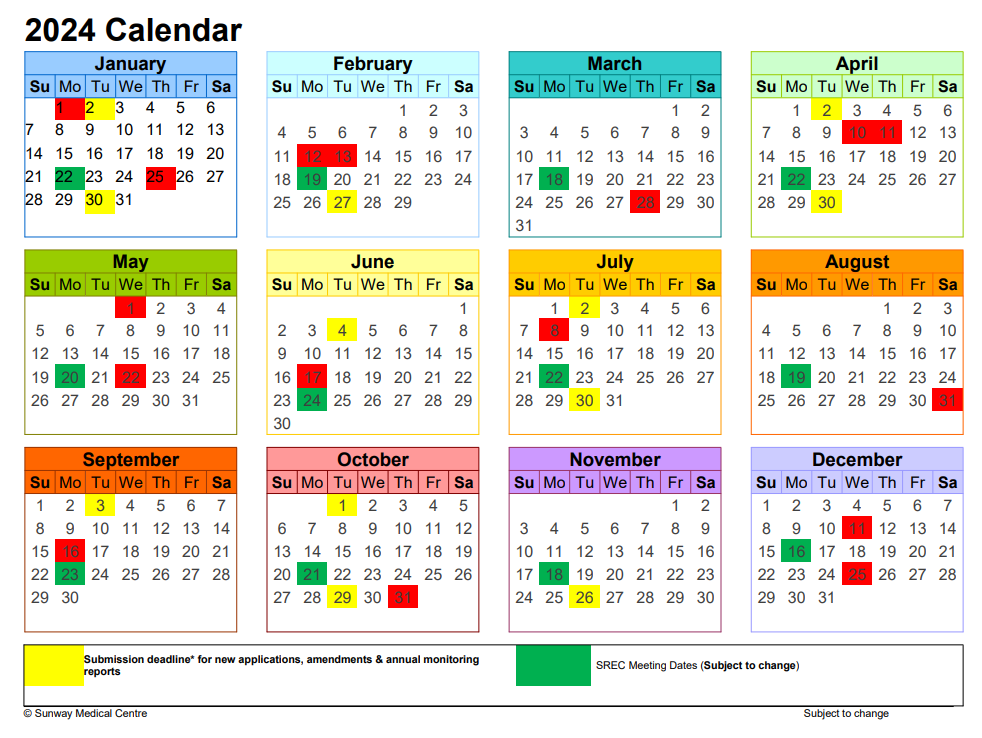
SREC Meeting Dates and Application Submission Due Date
Please click here to download the 2024 SREC meeting calendar. This calendar includes the meeting dates and application due dates. Applicant may also contact the SREC secretariat to confirm the SREC meeting dates and application due date.
Overview of Conducting Research in Sunway Medical Centre
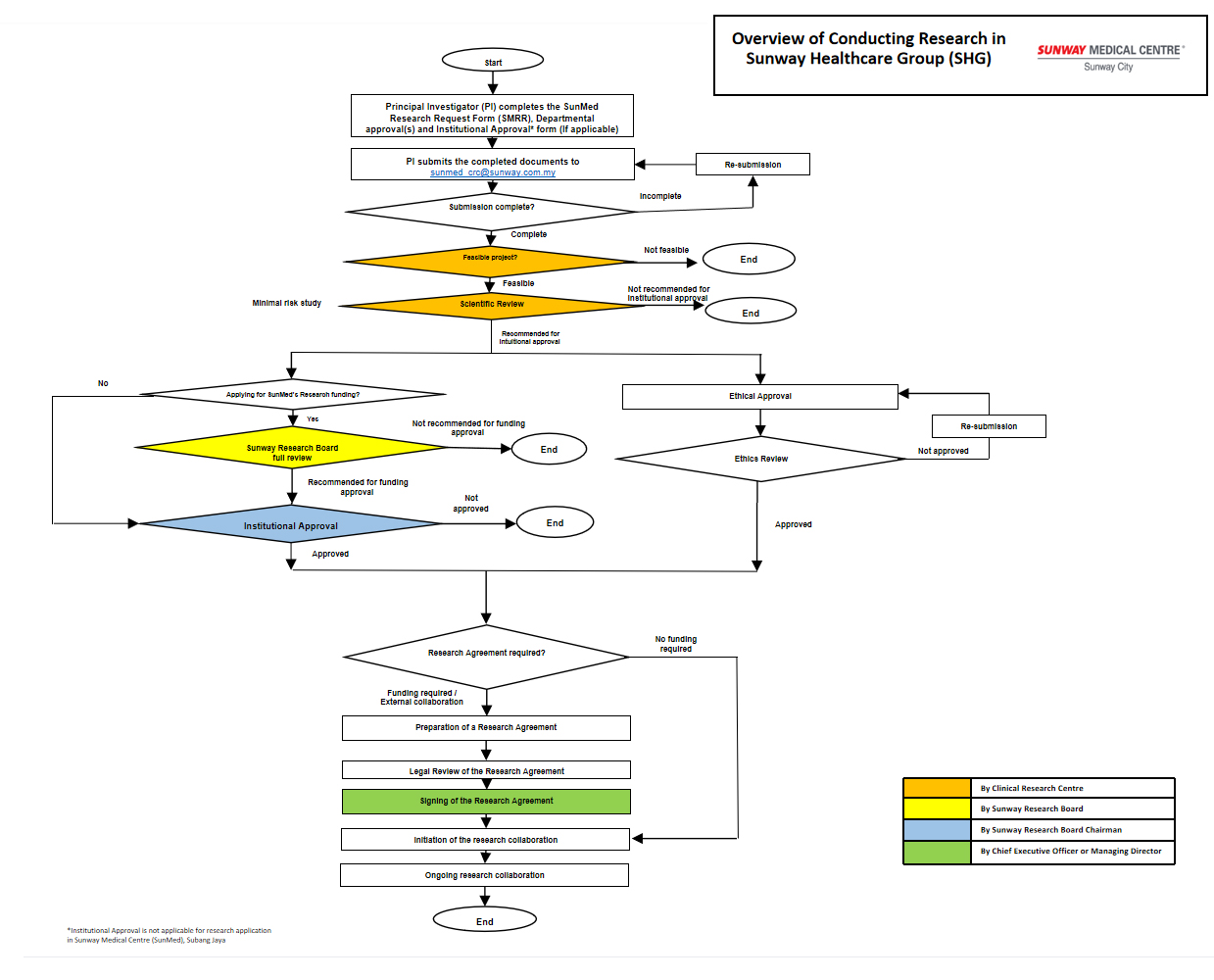
All research projects to be conducted in SunMed MUST obtain research ethic committee and institutional approvals.
Ethics Application
Please refer to SREC Application Guidelines on how to apply:
| SREC Form | Checklist |
|---|---|
| Research Amendment Application Form |
Informed Consent Form & Written Subject Information Checklist |
| Genetic Research Checklist | |
Institutional Approval
- SunMed Research Request Form (SMRR) completion
Principal investigator is required to complete the SunMed Research Request Form (SMRR) and read the Sunway Anti Bribery and Corruption (ABC) policies together with related CRC policies and procedures. Attach proposal and all written information that you are providing to research subject.
- Departmental Approval
Investigator has to approach the related department(s)’ Head for the approval(s). For instance, if your research involving nurses, then you are required to approach the Director of Nursing. If SunMed staff is the target subjects, then you should obtain the approval from Medical Director/Chief Executive Officer (CEO). If you are unsure which department to approach you may contact us.
Departmental Approval for SunMed
Sunway Medical Centre (SunMed) should be done via the link:- https://bit.ly/3ktQAwa
Submitting the SMRR form, Departmental Approval and a copy of your research proposal
Please submit the following to [email protected] :-
a) Signed and dated SMRR form
b) Signed and dated ABC declaration form (appendix of SMRR)
c) A copy of your recent research proposal with version and date
Kindly ensure all the documents are submitted to avoid delay of your research approval. Please take note that the department approval(s) is/are compulsory for feasibility assessment.

- Institutional Approval
This will commence after the completion of feasibility assessment and scientific review. If the project is approved by Sunway CRC Research Board (SRB) the investigator will receive an institutional approval letter.
For Sunway Medical Velocity (SMCV), you are required to complete the Institutional Approval Form.
- Research Agreement
Preparation and signing of collaborative research agreement
CRC will contact you if an agreement is required. We may provide the agreement template to the collaborator for review and comment. Once all parties agreed and signed the agreement then only the study could be initiated.
Research Reporting
i. Research Progress Report - Download Here
- Research under investigator initiated research initiated Research (IIR) is required to submit research report annually every 6 months
ii. End of Research (EOR) Report - Download Here
- Research under investigator initiated research initiated Research (IIR) is required to submit research report annually every 6 months
iii. Pre-publication Submission Review - Download Here
Send a copy of your drafted publication materials to [email protected] prior submission for publication. After publication a copy of the publication shall be submitted to delegated CRC staff. Furthermore, acknowledgement to Sunway Medical Centre is required for funded research. This applicable for any research project that conducted in Sunway Medical Centre.
Note: This ONLY applicable for research project conducted Sunway Medical Centre
Research Non-Compliance & Protocol Deviation Reporting
For Investigator Initiated Research (IIR) which does not involve investigational products (Clinical Trial), submit Research Noncompliance Form to CRC.
For Industry Sponsor Research (ISR) which involves investigational products (Clinical Trial), submit SREC Protocol Deviation Reporting Form.
Reporting Timeline - Minor within 30 days, Major within 7 days (calendar date).
Internship and Attachment
If you are interested to apply for attachment or internship at SunMed CRC, kindly send your application to [email protected] with the following documents:
- A cover letter addressing to Head of Division, Clinical Research and Medical Education, Sunway Medical Centre
- A copy of your resume
Operation hours
8.00 am - 5.30 pm
Inquiry
For any inquiry and feedback, please contact Clinical Research Center (CRC) at [email protected].
Contact Us
| Address | SunMed Clinical Research Centre, F-03-02, Block F, Sunway Geo Avenue, Jalan Lagoon Selatan, Bandar Sunway, 47500 Selangor |
| Tel No | +603 8601 1072 +603 8601 1079 +603 8601 1080 |
| Fax No | +603 8601 1069 |
| [email protected] [email protected] |
Accreditation

Australian Council on Healthcare Standards (ACHSI)

Malaysian Society for Quality in Health
Useful Links
National Medical Research Registry (NMRR)
Clinical Research Centre – Ministry of Health Malaysia (CRC-MOH)
Medical Device Authority (MDA)- Ministry of Health Malaysia
Guidelines for Stem Cell Research and Therapy – Ministry of Health Malaysia
Society of Clinical Research Professionals Malaysia (SCRPM)
National Committee for Clinical Research (NCCR)
National Pharmaceutical Regulatory Agency (NPRA)
ClinicalTrials.gov – Registry of Clinical Trials in U.S. and around the world